In 2014 and 2016, West Africa and other parts of the world experienced the largest and most complex Ebola outbreak since the discovery of the virus in 1976. This outbreak devastated the region, causing high consequences for the health system, the economy, and social unrest.
The outbreak also revealed poor capacities in clinical research and the need for regional scientists to collaborate and improve these capacities with a robust research agenda on epidemic diseases. It also brought opportunities like implementing clinical vaccine trials in these countries. These trials include the Prevail Vaccination and other ring vaccinations in Guinea and Sierra Leone. These clinical trials were conducted in Liberia, Guinea, and Sierra Leone and showed the potential impact of multi-country collaboration.
Following this, researchers and ministries of health from the different affected countries established a cross-country research institution, The West African Consortium on Clinical Research on Emerging Pathogens (WACCREP). The original mission of WACCREP is to improve capacities in clinical research to provide evidence to guide response-related activities. Since its inception, WACCREP has brought together research scholars from five countries, including Cote d’Ivoire, Guinea, Liberia, Mali, and Sierra Leone, who have researched endemic and emerging pathogens. WACCREP, to achieve its mission, works with several stakeholders to build the country’s capacity for pandemic preparedness and readiness. These include universities, non-governmental organizations, and national public health agencies.
Over the decade, the area has also notified sporadic cases of Ebola and Marburg and experienced several outbreaks of Lassa and COVID-19. More recently, the area has been experiencing Mpox, which the World Health Organization (WHO) has declared a Public Health Emergency of International Concern. This clearly illustrates the high risk of human exposure to epidemic diseases in West Africa and the increasing need to build capacities to unlock evidence and share knowledge between countries.
There has also been increased recognition by various global organizations that strengthening health systems is necessary to improve long-term regional and global health security and withstand outbreaks. Therefore, given the threat, WACCREP is a shared interest by Sierra Leone, Guinea, Liberia, Mali, and Cote d’Ivoire to advance regional preparedness for global health security by sharing regional research, best practices, and evidence to inform epidemic pathogen policies.
Objective of WAC -CREP
Objectives
To strengthen subregional collaboration for preparedness to mitigate epidemics through clinical research platform, harmonised framework and capacity building.
Specific Objectives
- Strengthen institutional capacity to facilitate the training and development of the next generation of clinical research scientists and health professionals to conduct high-quality clinical research to address global health security needs.
- Develop a sustainable institutional framework for translational clinical research, advocacy to disseminate and promote the utilization of findings to support epidemic surveillance through networking
- Promote community engagement to facilitate clinical research and epidemic preparedness, surveillance and response in the sub-region
- Establish a harmonized clinical research platform to strengthen ethical and regulatory procedures across the subregion
- Mobilize resources and respond to multi-country calls for proposals to enhance and sustain the collaborative research agenda.
Roles and Responsibilities of WAC-CREP
- Provide technical guidance and advice to member countries on prevention and response to outbreaks.
- Support individual countries in advocacy for budgetary needs
- Develop a common research agenda that aligns with the national health research for the sub-region
- Advocate for sustainable research capacity-building programs for researchers in the sub-region.
- Create and maintain the platform for research experience-sharing activities with full engagement of individual core team members of the consortium.
- Promote multi-country research grant support from the scientific community to the consortium.
- Advocate for strong political will from individual Governments.
- Identify research priority on epidemic pathogens.
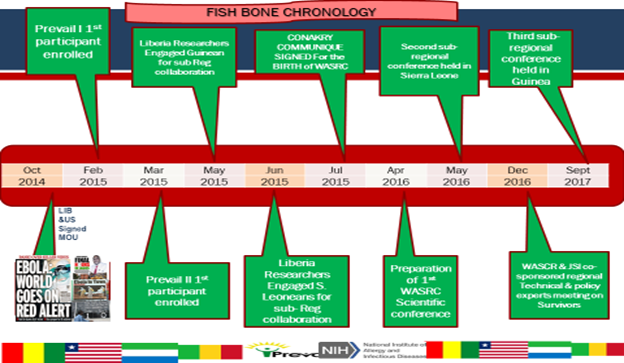
Summary of accomplishments since 2015
| · Agreement on a joint communique for the establishment of the Sub-Regional collaboration on clinical research
· The successful hosting of annual WACCREP Scientific Research conferences in Liberia, Sierra Leone, Guinea, Mali and Cote d’Ivoire · Official Launch of PREVAC (Partnership research for Ebola Virus Vaccine) studies in the Sub-Region at the WAC-CREP conference in Sierra Leone by INSERM and NIH. · Successful implementation of the Regional Transmission Prevention and Survivor Services Program held in Monrovia on 2 and 3 December 2016. · Launching of study to assess Gilead Science compound GS5734 for the eradication of persistent Ebola virus in Ebola Survivors, Liberia, and Guinea. · Research Agenda 2023· Grant submission to Delta Africa, WHO (Liberia, Mali, Guinea), Wellcome Trust, Science for Africa (EPSILON), Fogarty Research Training Grant (D71) · Completed PREVAC 2023 · Presentation at International Conference · Publication (Lancet, British Medical Journal American of Preventive Medicine and Public Health) · Grant Received (WHO Polio, Fogarty D43 Research Training Grant, Digital Health and Innovation, Bill & Melinda Gate) · Weekly and Quarterly Meeting |
Research Institutions and Universities
| Institutions | Country |
| National Public Health Institute of Liberia (NPHIL) | Liberia |
| Ministry of Health Liberia (MoH) | Liberia |
| Partnership for Research on Vaccines and Infectious Diseases in Liberia (PREVAIL) | Liberia |
| University of Liberia (UoL) | Liberia |
| University of Sciences, Techniques and Technology of Bamako (USTTB) The University Clinical Research Center (UCRC) and Malaria Research Training Center (MRTC) program | Mali |
| Ministry of Health of Mali | Mali |
| National Institute of Public Health Mali | Mali |
| Center for Vaccine Development, (CVD) | Mali |
| Center of Research and Biomedical Analysis (CRAM) Macenta | Guinea |
| National Center for Training and Research in Rural Health of Mafèrinyah (NCTRRH) | Guinea |
| National Institute of Public Health Guinea | Guinea |
| Gamal Abdel Nasser University of Conakry (UGAN) | Guinea |
| Ministry of Health of Guinea | Guinea |
| College of Medicine and Allied Health Sciences, (CMAHS) University of Sierra Leone | Sierra Leone |
| Ministry of Health | Sierra Leone |
| Fourah Bay College (FBC) | Sierra Leone |
| National Public Health Agency (NPHA) | Sierra Leone |
| University Felix Houphouet Boigny (UFHB) | Cote d’Ivoire |
| Ministry of Health and Public Hygiene and Universal Health Coverage | Cote d’Ivoire |
| Infectious Diseases and Associated Pathologies Research (CERMIPA) | Cote d’Ivoire |
| Pasteur Institute (PI) | Cote D’Ivoire |
| National Institute of Public Hygiene | Cote D’Ivoire |
| National Institute of Public Health | Cote D’Ivoire |
Problem statement
The West African region was affected by an unprecedented EVD outbreak that claimed more than 11,000 lives. The outbreak occurred in the midst of a weakened health system with limited research capacity and coordination platforms at the sub-regional level. Investment in sub-regional collaboration and research systems strengthening will contribute by providing technical support to member countries’ Ministries of Health and the National Public Health Institutes in preventing and averting the effects of subsequent outbreaks. Furthermore, there is a need for a sub-regional governance framework for the management of biological sample sharing, biosecurity, biosafety, and the use of existing regional biobanks.
Situational analysis using SWOT
Strength
There has been a significant amount of research expertise in the region within the last decade in the following areas: Clinical scientists, bioethicists, Public Health experts, and Biostatisticians, amongst others. Most of these experts in the affected countries in the subregion have taken charge of fighting the spread of outbreaks and other newfound research ideas. These experts are the ones who could foster collaborations on research and come together as a regional group and can be identified as our strength. Before this collaboration, there was inadequate coordination and regional corporations to address the epidemic burden effectively. Especially during the period of Ebola, countries most affected in the region were still having EVD transmission. Cognizant of this fact, the Leaders of these countries underscore the need for sub-regional and regional collaboration to combat and end the Ebola and subsequent outbreaks in West Africa.
We needed to harness our collective efforts by creating a common platform to strengthen our collective capabilities in clinical trials while, at the same time, providing the necessary support and care for EVD survivors and any other disease burden. Such platforms must be considered to effectively tackle emergencies for epidemics and contribute meaningfully to building a resilient healthcare system.
Weaknesses
Most countries in the sub-region have a weakened health system, and the need to strengthen them is not an overstatement. Overall, research capacity remains a significant obstacle, and any effort to alleviate the situation is a laudable way to address the low research capacity. The number of weaknesses identified are but not limited to the following:
- Limited funding for clinical research
- Lack of regional health information management system
- Limited awareness of the WACCREP body by member countries.
- Continued reliance on external funding with limited or non-existent domestic financing for research
- Not enough focus on research and development (R&D)
- Issues of common protocol and study design
- Ethical clearance procedures – Which approach: individual country review versus joint country-wide review.
- Lack of robust regulatory pathway
- Low-capacity building and no support structure for country ownership
- Weakened social mobilization and the role of the community in clinical research
- Lack of Governance for sample storage and sharing
Opportunity
The assurance of our country’s governments is to provide the will and political commitment and engage in a collaborative way of working together to arrest the continued scourge of the outbreak. Our working together with key stakeholders, which included the United States National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC); the Joint Liberia-U.S. Clinical Research Partnership for Research on Ebola Virus in Liberia (PREVAIL); the World Health Organization (WHO); the French National Institute of Health and Medical Research (INSERM); the London School of Hygiene and Tropical Medicine (LSHTM); African CDC, WAHO and the Health Ministries of the members countries involved, among others.
The opportunity for continuous professional learning education through training and capacity building will strengthen and solidify a strong collaboration. This is an opportunity we cannot afford to miss, as it provides a window of opportunity for solutions to emerging epidemic pathogens and strengthens our resolve to work together more than ever before to tackle not only EVD-like crises but other re-emerging infectious diseases in the region and the global world.
The partnership believes that this strategy creates an opportunity for current and future collaboration in transferring and/or sharing technologies (prevention, treatment, research, etc.) to control and eliminate EVD and other infectious disease epidemics from within the subregion.
It is important to recognize these opportunities and take advantage of them. These opportunities include but are not limited to:
- Building research capacity development within the sub-region and globally.
- Continuous engagement with government to enhance effective collaboration, Coordination, and Cooperation with partners.
- Enhancing WACCREP recognition by parent institutions like the Mano River Union, WAHO, and other institutions
- Increased strength of community involvement and harnessing their sensitization capacity in fostering research in the sub-region
- Strengthening the governance structure to increase accountability and reduce “disconnectedness” between governments and the research community.
Threat
Limited research capacity has weakened the health system, population growth, and mobility, and climate change remains a potential threat to the region. It will remain a challenge to global health security. As much as this is the case, WACCREP needs to implement purposeful measures or steps to mitigate the negative impact of weakened health systems. On the other hand, there are threats that WACCREP has little or no control over, but that shouldn’t mean that WACCREP is helpless. We can minimize the negative impact of these situations by using our strength and unity as a laudable group. Familiar threats where WACCREP has no, or little control include these but are not limited to:
- Government economic recession resulted in a lack of funding, the non-prioritizing building of research capacity and not providing funding support.
- Climate change could negatively impact human health by transmitting new and reemerging pathogens across the human, animal, and environmental landscape.
- New and increased migration trends because of cross-border and population growth
- Poor Water, sanitation, and hygiene conditions, including low socioeconomic standards.
- Limited interest from countries’ Ministries of Health
- Limited support from regional bodies, e.g.: Mano River Union, WAHO, ECOWAS
- Limited support from global health and research bodies
Strategic Priorities and Activities
Based on WACCREP’s Mission, Vision, Values, achievements over its 10 years of existence, and current situation analysis, it is important for us to set strategic objectives and activities for the next 5 years: 2024-2028.
Strategic Objective 1:
Strengthen institutional capacity to facilitate the training and development of the next generation of clinical research scientists and health professionals to conduct high-quality clinical research to address global health security needs.
Strengthen the capacity of institutions in implementing clinical research.
Summary Activities
- Conduct a human capacity building needs assessment and identify areas for capacity building within countries.
- Mobilize resources for research training for young researchers
- Identify funding opportunity
- Create and maintain a framework for exchanges with ministries of health and other stakeholders.
- Establish and maintain specialized repositories for research products
- Plan and host, on a rotational basis, the WACCREP bi-annual conference.
Strategic Objective 2:
Develop a sustainable institutional framework for translational clinical research, advocacy to disseminate and promote the utilization of findings to support epidemic surveillance through networking
Summary Activities
- Do a mapping of Research Institutions, donors and partners for research within the WAC member states
- Organize periodic webinars to attract internal and external collaborators
- Develop and strengthen WAC-CREP communication strategies
- Facilitate resource sharing among member countries
Strategic Objective 3:
Promote community engagement to facilitate clinical research and epidemic preparedness, surveillance and response in the sub-region
Summary Activities
- Raise community awareness of clinical research activities
- Train volunteers to encourage communities to report cases
- Create epidemic preparedness and response groups
- Establish mentoring programs to promote community engagement
Strategic Objective 4:
Facilitate clinical research platform to strengthen management, ethical, and regulatory procedures across the subregion
Summary Activities
- Invest in continuous ethical/regulatory and management capacity strengthening for research,
- Increase awareness of member states ethical/regulatory and management practices.
- Provide training, guidance, and regulatory procedures to cross-countries
- Develop and update Ethics/Regulatory policy.
- Provide internal study monitoring within member countries
- Develop Research Management Procedure Standard
- Identify barriers that delay regulatory and ethical approvals in member countries.
- Develop and propose evidence-driven regulatory and ethical procedures to mitigate barriers.
- Analyze relevant regulations across countries.
WAC-CREP Strategic Plan (2024-2028)
| Objective 1: Strengthen institutional capacity to facilitate the training and development of the next generation of clinical research scientists and health professionals to conduct high-quality clinical research to address global health security needs | ||||||
| Timeline | Estimated Cost | |||||
| Strategies | 2024 | 2025 | 2026 | 2027 | 2028 | |
| Set up of WAC-CREP secretariat with office and staffs for the smooth implementation of WAC-CREP strategic and operational plan | X | X | ||||
| Establish a robust financial and grant management within WAC-CREP regional office | X | X | X | |||
| Conduct an Institution (Laboratory, research institutions, medical centers, etc.) needs assessments within the WAC-CREP region member countries. | X | X | ||||
| Conduct a human capacity building needs assessment and identify areas for capacity building within countries | X | X | ||||
| Establish WAC-CREP research centers offices for WAC in member countries | X | X | ||||
| Facilitate ethical/regulatory clearance procedures for member countries, training on ethical clearance/regulatory | X | X | ||||
| Capacity building for infrastructure; Create networks of laboratory capacity with member countries | X | X | X | X | X | |
| Network with regional and global research bodies | X | X | X | X | X | |
| Develop and run fellowships for young researchers to develop research capacity/leadership; Encourage young researchers by awarding research grants | X | X | X | X | X | |
| Identify opportunities for leadership capacity on research | X | X | X | X | X | |
| Develop a comprehensive capacity building plan for both human and institutions within the WAC-CREP member states for the conduct of research | X | X | ||||
| Implement the Capacity Building Plan for both human and institutions | X | X | X | X | X | |
| Establish an equipment maintenance and renewal plan | X | X | X | X | X | |
| Develop the M&E Framework and use it to monitor and evaluate the implementation of the Strategic Plan | X | X | X | X | X | |
| Strengthen the mentoring system for young researchers | X | X | X | X | X | |
| Objective 2: Develop a sustainable institutional framework for translational clinical research, advocacy to disseminate and promote the utilization of findings to support epidemic surveillance through networking | ||||||
| Timeline | Estimated Cost | |||||
| Strategies | 2024 | 2025 | 2026 | 2027 | 2028 | |
| Do a mapping of Research Institutions, donors and partners for research within the WAC-CREP member states |
X |
X |
X | X | X | |
| Identify funding sources for research by various means including using link such as https://globalhealthtrials.tghn.org/funding/ | X | X | X | X | X | |
| Establish scientific committee for drafting research and training projects | X | |||||
| prepare and submit solicited proposal in response to « Calls for Proposals well as submitting unsolicited research projects | X | X | X | X | X | |
| Organize periodic webinars to attract internal and external collaborators | X | X | X | X | X | |
| Publicize the WAC-CREP through scientific publications, forums and symposia | X | X | X | X | X | |
| Revise and strengthen WAC-CREP communication strategies | X | X | X | X | X | |
| Objective 3: Promote community engagement to facilitate clinical research and epidemic preparedness, surveillance and response in the sub-region | ||||||
| Timeline | Estimated Cost | |||||
| Strategies | 2024 | 2025 | 2026 | 2027 | 2028 | |
| Strengthen partnerships with local and regional organizations | X | X | X | X | X | |
| Strengthen media engagement to make WAC-CREP more visible | X | X | X | X | X | |
| Increase country and regional profile of WAC-CREP | X | X | X | X | X | |
| Identify WAC-CREP champions within countries for increased visibility and resource mobilization | X | |||||
| Host Regional Scientific Research Conference, Symposium and Research fairs (booths) | X | X | X | X | X | |
| Encourage the hosting of research career day on the university campus in member countries. | X | X | X | X | X | |
| Promote Annual publication of research by WACCREP members | X | X | X | X | X | |
| Objective 4: Facilitate clinical research platform to strengthen management, ethical, and regulatory procedures across the subregion | ||||||
| Timeline | Estimated Cost | |||||
| Strategies | 2024 | 2025 | 2026 | 2027 | 2028 | |
| Set up a publication and archiving committee | X | X | ||||
| Animate, update and sustain the WAC-CREP website other media platforms | X | X | X | X | X | |
| Create and maintain a framework for exchanges with ministries of health and other stakeholders | X | X | X | X | X | |
| Establish and maintain specialized repositories for research products | X | |||||
| Established a scientific publication platform. | X | |||||
| Plan and host, on a rotational basis, the WACCREP bi-annual conference. | X | X | X | |||
Measures of Success by 2028
WACCREP Monitoring framework
- Official recognition by member states MoH and Ministries of Higher Education.
- Number of regional and national meetings organized or attended.
- Number of collaborative grants obtained.
- Number of scientific publications or presentation achieved by member institutions (define eligibility criteria for a publication)
- Number of new countries adhering to the consortium
- Number of individuals trained in (Short, master, and PhD programs)
- Number Research Study conducted
Organizational Structure
Local Countries Organigram
Define governance structure – Identify roles and responsibilities of each group and individual involved
- Identify the groups and individuals involved in governance and clarify the roles of each in carrying out the major functions of governance. Likely participants in governance include:
-
Governing and Advisory Board
-
Executive/Steering Committee
-
Scientific Advisory Board
-
Executive Chair
-
Executive Secretariat
-
National Secretariat
-
Governance Advisory Board
|
Committee |
Skills required |
Responsibilities |
Recommendations |
| Governance Advisory Board (GAB):
|
§ External Experienced leader or advisors; politically savvy; good communicators.
§ scientific experts; global connections; instinct and experience.
|
§ Advise, offer guidance and expertise
§ Set policies, oversee, ensure compliance with mission § Provide recommendations § Make decision within their scope |
MoH, MoHE
Directors of Research Institute Rector/University President
|
Executive Steering Committee
|
Committee |
Skills required |
Responsibilities |
Recommendations |
| Executive Steering
Committee (ESC):
|
§ Experienced Scientist mentors and advisors.
§ Global connections; instinct and experience. § Capacity to get grants
|
|
Co-Chairs:
|
Scientific Advisory Board
| Committee | Skills required | Responsibilities | Recommendations: |
| 1. Scientific Advisory Board (SAB):
|
Scientific leadership; strategic scientific vision; global /public health experience.
|
Primarily responsible for scientific vision:
|
SSC Chair:
Scientific representatives:
|
Executive Secretariat
|
Committee |
Skills required |
Responsibilities |
Recommendations: |
| Executive Secretariat (Chair)
|
Operational leadership; experience in implementing global /public health clinical research and other diseases.
|
Primarily responsible for operational vision:
|
Executive Chair:
|
WAY FORWARD
- Finalize Governance Structure
- Set up the operational Secretariat (Executive Secretary, Assistants, and other positions)
- Select a WAC-CREP Champion
- Political endorsement from each country MoH
- Letter to Country MoH advocating for WACCREP,
- letter to WAHO leadership, CC: MRU)
- Develop a Yearly Operational plan
- Develop Monitoring Plan
The vision of WAC -CREP
Vision
WACCREP’s vision is to save lives and contribute to improving and maintaining global health security through cross-country collaborative clinical research on epidemics and pathogens.
Mission
Advance regional preparedness for global health security through collaborative research, sustainable research capacity, and evidence-based health policy. This will enable an effective response to future pandemics.
Stakeholders
Goverments :
- Republic of Liberia
- Republic of Sierra Leone
- Republic of Guinea
- Republic of Mali
- Republic of Côte d’Ivoire
Regional Affiliates :
- Mano River Union (MRU)
- West African Health Organization (WAHO)
- Africa Center for Disease Control (AfCDC)
- Academic Institutions (Universities) with Research Portfolio in the region
- World Health Organization (WHO)
Partners and Agencies:
-
- National Institute of Allergy and Infectious Disease (NIAID), National Institute of Health
- National Institute of Health and Medical Research (INSERM)
- John Snow Institute (JSI)
- National Public Health Institute of Liberia (NPHIL)
- Scientific Research Center of Suisse (CSRS)Cote d’Ivoire
- Africa One Health University Networks (AFROHUN)
- The Global Health Network (TGHN)
- Tropical Disease Research Special Project (TDR)
- West African Research Network in Infectious Diseases (WARN-ID)
- Social Marketing Agency of Cote d’Ivoire
- International entities: The World Bank; World Health Organization.
Values :
- Collaboration
- Integrity
- Leadership
- Transparency
- Urgency
- Capacity building
- Knowledge sharing
- Technical Excellence
- Shared culture
- Accountability